The Dynamic Duo: How DNA and Epigenetics Shape Health and Disease
In the grand and intricate tapestry of human life, two fundamental forces are at play: DNA, the immutable blueprint, and epigenetics, the dynamic interpreter of that code. Together, they provide the instructions and the regulatory fine-tuning for every cell, influencing everything from our physical appearance and inherited traits to our susceptibility to disease. While DNA holds the static genetic instructions passed down through generations, epigenetics serves as the crucial interface between our genes and our environment, dictating which parts of the blueprint are read and when.
This dual system—one of a fixed code, the other of a flexible regulatory layer—is fundamental to understanding how health and disease manifest in the human body. As we delve deeper into the complex relationship between DNA and epigenetics, we are uncovering new truths about human development, aging, and the origins of some of our most devastating illnesses.
The Blueprint of Life: DNA and Its Imperfections
At the heart of every cell lies the human genome, a vast library of approximately 3 billion base pairs of DNA. Within this genetic expanse are roughly 20,000 protein-coding genes, which carry the instructions for building and maintaining the human body. However, the majority of our DNA is not a simple coding sequence; it is a complex landscape of regulatory elements, non-coding RNAs, and once-dismissed “junk” sequences that are now known to be vital for fine-tuning gene expression.
The integrity of this blueprint is paramount. Inherited variations, such as mutations, insertions, deletions, and copy number variations, can disrupt gene function or regulation. When these changes occur, they can lead to a wide spectrum of diseases, ranging from single-gene disorders like cystic fibrosis and Huntington’s disease to complex, multifactorial conditions like heart disease and diabetes. In recent years, technological revolutions in DNA sequencing and gene editing, particularly with the advent of tools like CRISPR-Cas9, have given us unprecedented power to identify these genetic flaws and, in some cases, correct them. Yet, the presence of a genetic predisposition does not always guarantee the manifestation of a disease, a paradox that can only be explained by the second, dynamic layer of genetic control: epigenetics.
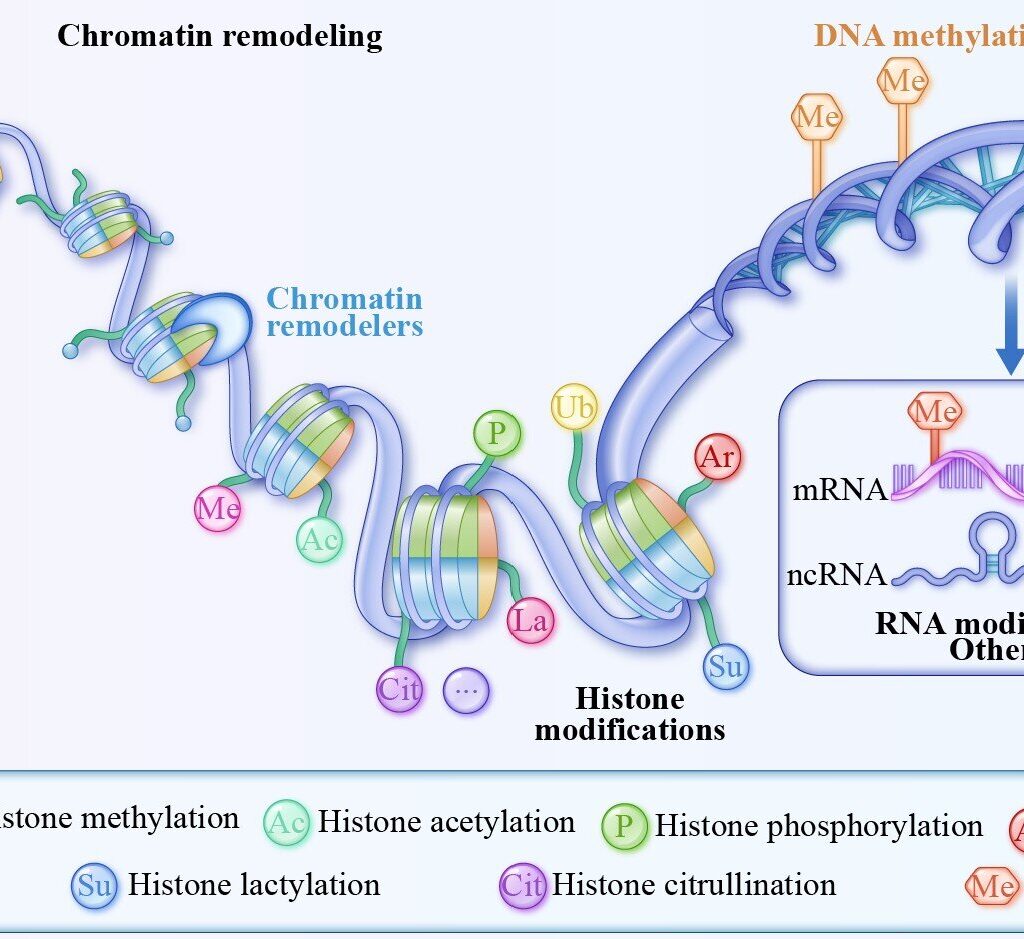
The Interpreter: Molecular Mechanisms of Epigenetic Regulation
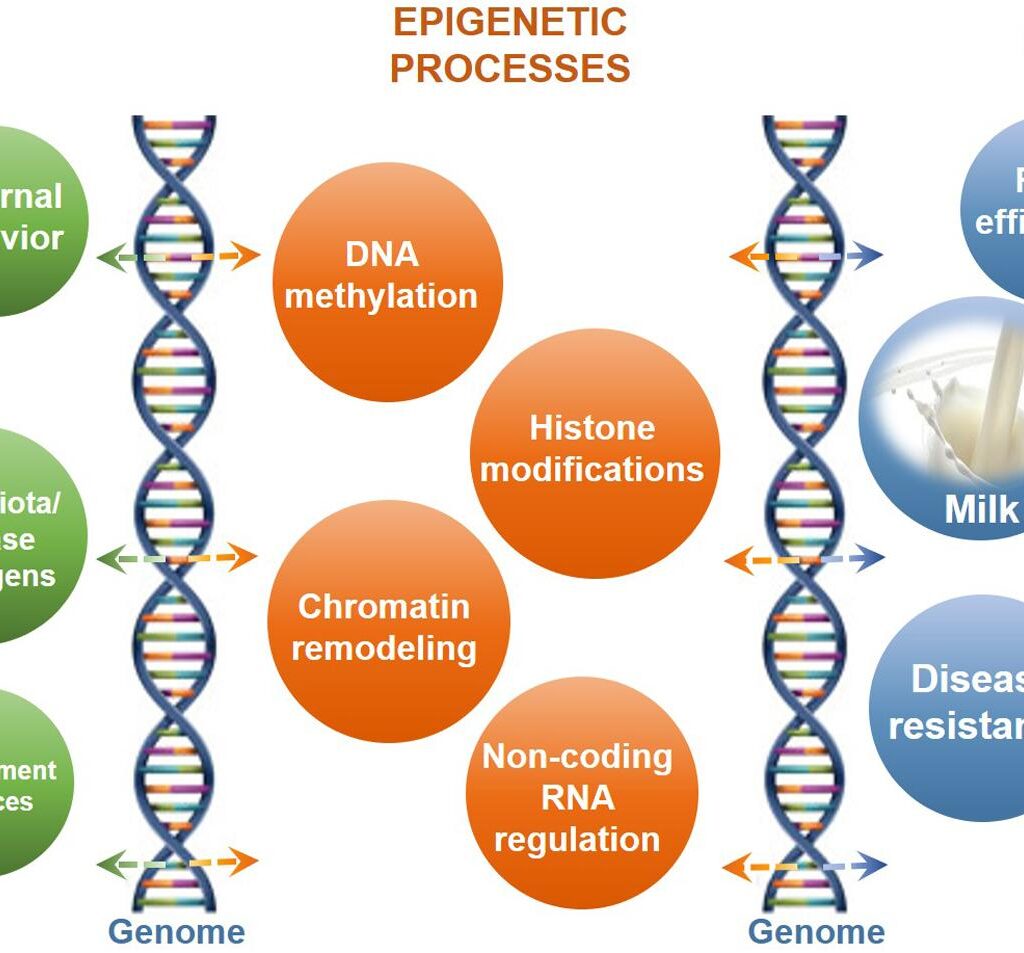
Epigenetics refers to a set of heritable changes in gene expression that do not involve alterations to the underlying DNA sequence. Instead, these modifications act as a sophisticated regulatory code, instructing the cell’s machinery which genes to switch on or off. The major mechanisms of epigenetic regulation include:
DNA Methylation: This process involves the addition of a methyl group to a cytosine base, typically at regions known as CpG islands within a gene’s promoter. Like a molecular “sticky note,” this methylation mark can effectively silence a gene, preventing the cellular machinery from reading it. In a developing embryo, this mechanism is crucial for ensuring that genes for a heart cell are not activated in a developing nerve cell. However, in disease states like cancer, this process can go awry, leading to the silencing of critical tumor suppressor genes.
Histone Modification: DNA is not free-floating within the nucleus; it is wrapped tightly around proteins called histones, which act as molecular spools. The tightness of this wrapping is crucial for gene accessibility. Chemical tags—such as acetyl, methyl, or phosphate groups—can be added to or removed from the histone “tails.” For example, adding acetyl groups (acetylation) can loosen the DNA’s grip on the histones, making the gene accessible for transcription. Conversely, the removal of these groups can cause the DNA to coil more tightly, effectively shutting the gene down. This elegant system allows cells to rapidly alter gene expression in response to internal and external cues.
Non-coding RNAs: The majority of our genome does not code for proteins. Instead, it produces a variety of non-coding RNA molecules, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). These molecules play a powerful role in gene regulation by acting as post-transcriptional messengers, fine-tuning gene expression and influencing everything from cell differentiation to disease progression.
Chromatin Remodeling: All of these mechanisms are part of a larger process known as chromatin remodeling, which involves ATP-dependent complexes that can physically adjust the DNA-histone interactions. This system ensures that the genome’s architecture is constantly being reorganized to support the specific needs and identity of each cell type.
These epigenetic modifications are dynamic and reversible, offering a stark contrast to the permanent nature of DNA mutations. They provide a flexible and responsive layer of control that allows the same static genetic blueprint to create the diverse array of cell types that make up a complex organism.
The Environmental Bridge: Lifestyle, Experience, and Epigenetic Plasticity
Perhaps the most compelling aspect of epigenetics is its role as a bridge between our genes and our environment. Unlike most genetic mutations, epigenetic changes can be directly induced by a wide range of external factors. Our diet and nutritional intake, our exposure to chemical pollutants, our level of physical activity, and even our psychological state and stress levels can all leave their mark on the epigenome.
This phenomenon, known as epigenetic plasticity, allows organisms to adapt to their surroundings. For instance, studies have shown that a high-fat diet can alter methylation patterns in genes related to metabolism, contributing to the development of obesity and type 2 diabetes. Similarly, chronic psychological stress can lead to enduring changes in histone modifications in genes linked to the stress response system, potentially increasing the risk for mood disorders.
The impact of these environmental influences can even transcend generations. This is a concept known as transgenerational epigenetic inheritance, where environmental exposures can induce epigenetic changes in germ cells, affecting the health and disease risk of offspring for generations to come. This discovery is reshaping our understanding of heritability, suggesting that our ancestors’ experiences can, in a very real way, be passed down through the generations.
The Epigenetic Link to Disease: Mechanisms and Examples
Epigenetic dysregulation is no longer seen as a mere consequence of disease but as a critical driver of its onset and progression.
Cancer: This is the field where the role of epigenetics is perhaps best understood. In a cancer cell, two major methylation changes occur: promoter hypermethylation and global hypomethylation. Promoter hypermethylation, a hallmark of cancer, involves the excessive methylation and silencing of tumor suppressor genes like p16 and BRCA1, which normally act as brakes on cell growth and division. Simultaneously, cancer cells often exhibit genome-wide hypomethylation, which can activate oncogenes and promote genomic instability, further fueling tumor growth and metastasis.
Neurological Disorders: The brain’s complex functions are heavily reliant on proper gene expression, and thus, neurological diseases are often tied to epigenetic dysregulation. In conditions like Alzheimer’s and Parkinson’s, aberrant DNA methylation and histone acetylation affect genes involved in neural cell health and synaptic plasticity. Similarly, in trinucleotide repeat disorders like Fragile X syndrome and Huntington’s disease, an increase in CpG methylation at expansion sites influences the age of onset and disease progression.
Metabolic and Cardiovascular Diseases: The growing epidemics of obesity, diabetes, and atherosclerosis are deeply intertwined with epigenetic imbalances. Dietary influences can modulate DNA methylation in genes linked to metabolism, inflammation, and insulin signaling. These epigenetic changes can alter how our bodies process food and store fat, laying the groundwork for chronic metabolic disease.
The Horizon: Epigenetic Therapies and Precision Medicine
The reversibility of epigenetic changes presents a compelling opportunity for new therapeutic strategies. Unlike genetic mutations, which are permanent and difficult to correct, epigenetic marks can be altered with drugs and other interventions. This has led to the development of novel therapies that target the enzymes responsible for epigenetic modifications.
Already, drugs known as DNA methyltransferase (DNMT) inhibitors and histone deacetylase (HDAC) inhibitors have received FDA approval for the treatment of certain hematologic cancers. These drugs work by reversing the aberrant methylation and histone modifications that silence tumor suppressor genes, effectively reactivating the cell’s natural anti-cancer defenses. The next frontier in this field involves the use of advanced tools like CRISPR/dCas9, which can be engineered to specifically target and edit epigenetic marks at precise locations in the genome, offering the possibility of highly targeted and personalized epigenetic interventions.
In the future, the interplay between the genetic code and its epigenetic regulators will form the basis of a new era of personalized and preventive medicine. By profiling both an individual’s DNA sequence and their epigenetic marks, clinicians will be able to perform highly accurate disease risk assessments, tailor therapeutic strategies to each patient, and make individualized lifestyle recommendations.

In conclusion, DNA and epigenetics are two intertwined dimensions shaping health and disease. DNA provides the static, heritable blueprint, while epigenetics serves as its flexible, environmentally responsive interpreter. As we continue to unravel the complexities of their relationship, we are not only gaining a more complete understanding of disease mechanisms but also empowering ourselves with the knowledge to prevent, manage, and ultimately cure illnesses in the 21st century.